UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM
| QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For
the quarterly period ended
OR
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission
file number:
(Exact name of registrant as specified in its charter)
| (State or Other Jurisdiction of | (I.R.S. Employer | |
| Incorporation or Organization) | Identification No.) | |
| (Address of Principal Executive Office) | (Zip Code) |
Registrant’s
telephone number, including area code:
Indicate
by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange
Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2)
has been subject to such filing requirements for the past 90 days.
Indicate
by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule
405 of Regulation S-T (Sec.232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was
required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| ☒ | Smaller reporting company | ||
| Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate
by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ☐ No
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Trading Symbol(s) | Name of each exchange on which registered | ||
The
(The Nasdaq Capital Market) |
As of August 14, 2024, the number of outstanding shares of the registrant’s common stock, par value $ per share, was approximately .
COCRYSTAL PHARMA, INC.
FORM 10-Q FOR THE QUARTER ENDED JUNE 30, 2024
INDEX
| 2 |
Part I – FINANCIAL INFORMATION
COCRYSTAL PHARMA, INC.
CONDENSED CONSOLIDATED BALANCE SHEETS
(in thousands, except per share data)
| June 30, 2024 | December 31, 2023 | |||||||
| (unaudited) | ||||||||
| Assets | ||||||||
| Current assets: | ||||||||
| Cash | $ | $ | ||||||
| Restricted cash | ||||||||
| Tax credit receivable | ||||||||
| Prepaid expenses and other current assets | ||||||||
| Total current assets | ||||||||
| Property and equipment, net | ||||||||
| Deposits | ||||||||
| Operating lease right-of-use assets, net (including $ | ||||||||
| Total assets | $ | $ | ||||||
| Liabilities and stockholders’ equity | ||||||||
| Current liabilities: | ||||||||
| Accounts payable and accrued expenses | $ | $ | ||||||
| Current maturities of operating lease liabilities (including $ | ||||||||
| Total current liabilities | ||||||||
| Long-term liabilities: | ||||||||
| Operating lease liabilities (including $ | ||||||||
| Total long-term liabilities | ||||||||
| Total liabilities | ||||||||
| Commitments and contingencies | ||||||||
| Stockholders’ equity: | ||||||||
| Common stock, $ a par value: and shares authorized as of June 30, 2024, and December 31, 2023; shares issued and outstanding as of June 30, 2024 and December 31, 2023 | ||||||||
| Additional paid-in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Total stockholders’ equity | ||||||||
| Total liabilities and stockholders’ equity | $ | $ | ||||||
See accompanying notes to condensed consolidated financial statements.
| F-1 |
COCRYSTAL PHARMA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(unaudited)
(in thousands, except per share data)
| Three months ended June 30, | Six months ended June 30, | |||||||||||||||
| 2024 | 2023 | 2024 | 2023 | |||||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development | ||||||||||||||||
| General and administrative | ||||||||||||||||
| Total operating expenses | ||||||||||||||||
| Loss from operations | ( | ) | ( | ) | ( | ) | ( | ) | ||||||||
| Other income (expense): | ||||||||||||||||
| Interest income, net | ||||||||||||||||
| Foreign exchange gain (loss) | ( | ) | ( | ) | ( | ) | ||||||||||
| Total other expense, net | ||||||||||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ( | ) | ( | ) | ||||||
| Net loss per common share, basic and diluted | $ | ) | $ | ) | ) | ) | ||||||||||
| Weighted average number of common shares outstanding, basic and diluted | ||||||||||||||||
See accompanying notes to condensed consolidated financial statements.
| F-2 |
COCRYSTAL PHARMA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(unaudited)
(in thousands)
| Common Stock | Additional Paid-in | Accumulated | Total Stockholders’ | |||||||||||||||||
| Shares | Amount | Capital | Deficit | Equity | ||||||||||||||||
| Balance as of December 31, 2023 | $ | $ | $ | ( | ) | $ | ||||||||||||||
| Stock-based compensation | - | |||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||
| Balance as of March 31, 2024 | $ | $ | $ | ( | ) | $ | ||||||||||||||
| Stock-based compensation | - | |||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||
| Balance as of June 30, 2024 | $ | $ | $ | ( | ) | $ | ||||||||||||||
| Common Stock | Additional Paid-in | Accumulated | Total Stockholders’ | |||||||||||||||||
| Shares | Amount | Capital | Deficit | Equity | ||||||||||||||||
| Balance as of December 31, 2022 | $ | $ | $ | ( | ) | $ | ||||||||||||||
| Stock-based compensation | - | |||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||
| Balance as of March 31, 2023 | $ | $ | $ | ( | ) | $ | ||||||||||||||
| Stock-based compensation | - | |||||||||||||||||||
| Sale of common stock, net of transaction costs | ||||||||||||||||||||
| Net loss | - | ( | ) | ( | ) | |||||||||||||||
| Balance as of June 30, 2023 | $ | $ | $ | ( | ) | $ | ||||||||||||||
See accompanying notes to condensed consolidated financial statements.
| F-3 |
COCRYSTAL PHARMA, INC.
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(unaudited)
(in thousands)
| Six months ended June 30, | ||||||||
| 2024 | 2023 | |||||||
| Operating activities: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization expense | ||||||||
| Stock-based compensation | ||||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and other current assets | ||||||||
| Deposits | ||||||||
| Tax credit receivable | ( | ) | ( | ) | ||||
| Decrease in right of use assets | ||||||||
| Accounts payable and accrued expenses | ( | ) | ||||||
| Operating lease liabilities | ( | ) | ( | ) | ||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| Investing activities: | ||||||||
| Purchases of property and equipment | ( | ) | ( | ) | ||||
| Net cash used in investing activities | ( | ) | ( | ) | ||||
| Financing activities: | ||||||||
| Payments on finance lease liabilities | ( | ) | ||||||
| Proceeds from sale of common stock, net of transaction costs | ||||||||
| Net cash used in financing activities | ||||||||
| Net decrease in cash and restricted cash | ( | ) | ( | ) | ||||
| Cash and restricted cash at beginning of period | ||||||||
| Cash and restricted cash at end of period | $ | $ | ||||||
See accompanying notes to condensed consolidated financial statements.
| F-4 |
COCRYSTAL PHARMA, INC.
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE SIX MONTHS ENDED JUNE 30, 2024 AND 2023
(unaudited)
1. Organization and Business
Cocrystal Pharma, Inc. (“we”, the “Company” or “Cocrystal”), a clinical stage biopharmaceutical company incorporated in Delaware, has been developing novel technologies and approaches to create first-in-class or best-in-class antiviral drug candidates. Our focus is to pursue the development and commercialization of broad-spectrum antiviral drug candidates that will transform the treatment and prophylaxis of viral diseases in humans. By concentrating our research and development efforts on viral replication inhibitors, we plan to leverage our infrastructure and expertise in these areas.
The Company’s activities since inception have principally consisted of acquiring product and technology rights, raising capital, and performing research and development. Successful completion of the Company’s development programs, obtaining regulatory approvals of its products and, ultimately, the attainment of profitable operations is dependent on future events, including, among other things, its ability to access potential markets, secure financing, develop a customer base, attract, retain and motivate qualified personnel, and develop strategic alliances. Through June 30, 2024, the Company has primarily funded its operations through equity offerings.
Liquidity
The Company’s consolidated financial statements
are prepared using generally accepted accounting principles in the United States of America applicable to a going concern, which contemplates
the realization of assets and the satisfaction of liabilities in the normal course of business. The Company has incurred net losses and
negative operating cash flows since inception. For the six months ended June 30, 2024, the Company recorded a net loss of approximately
$
On June 30, 2024, the Company had cash and cash equivalents
of approximately $
The Company’s activities since inception have principally consisted of acquiring product and technology rights, raising capital, and performing research and development. Successful completion of the Company’s development programs, obtaining regulatory approvals of its products and, ultimately, the attainment of profitable operations is dependent on future events, including, among other things, its ability to access potential markets, secure financing, develop a customer base, attract, retain and motivate qualified personnel, and develop strategic alliances. Through June 30, 2024, the Company has primarily funded its operations through equity offerings.
The Company will need to continue obtaining adequate capital to fund operating losses until it becomes profitable. The Company can give no assurances that the additional capital it is able to raise, if any, will be sufficient to meet its needs, or that any such financing will be obtainable on acceptable terms. Our future cash requirements, and the timing of those requirements, will depend on a number of factors, including economic conditions, the approval and success of our products in development, the continued progress of research and development of our product candidates, the timing and outcome of clinical trials and regulatory approvals, the costs involved in preparing, filing, prosecuting, maintaining, defending, and enforcing patent claims and other intellectual property rights, the status of competitive products, the availability of financing, our success in developing markets for our product candidates and legal proceedings that may arise. We have historically not generated sustained positive cash flow and if we are not able to secure additional funding when needed, we may have to delay, reduce the scope of, or eliminate one or more of our clinical trials or research and development programs. If the Company is unable to obtain adequate capital, it could be forced to cease operations or substantially curtail its drug development activities. The Company expects to continue incurring substantial operating losses and negative cash flows from operations over the next several years during its pre-clinical and clinical development phases.
2. Basis of Presentation and Significant Accounting Policies
Basis of Presentation
The accompanying condensed consolidated financial statements have been prepared in accordance with United States generally accepted accounting principles (“U.S. GAAP”) for interim financial information, the instructions to Form 10-Q and Article 10 of Regulation S-X set forth by the Securities and Exchange Commission (“SEC”). They do not include all of the information and notes required by U.S. GAAP for complete financial statements. In the opinion of management, all adjustments (consisting of normal recurring accruals) considered necessary for a fair presentation have been included. The results of operations for the interim periods presented are not necessarily indicative of the results of operations for the entire fiscal year. For further information, refer to the consolidated financial statements and footnotes thereto included in the Company’s annual report on Form 10-K for the year ended December 31, 2023 filed on March 28, 2024 (“Annual Report”).
Principles of Consolidation
The consolidated financial statements include the accounts of Cocrystal Pharma, Inc. and its wholly owned subsidiaries: Cocrystal Discovery, Inc., Cocrystal Pharma Australia Pty Ltd. (“Cocrystal Australia”), RFS Pharma, LLC and Cocrystal Merger Sub, Inc. Intercompany transactions and balances have been eliminated. Cocrystal Discovery, Inc. conducts all of the Company’s research and development activities and oversees ongoing clinical trials conducted by others. Cocrystal Australia operates clinical trials in Australia. The other two subsidiaries are inactive.
Segments
The
Company operates in only
| F-5 |
Use of Estimates
Preparation of the Company’s consolidated financial statements in conformance with U.S. GAAP requires the Company’s management to make estimates and assumptions that impact the reported amounts of assets, liabilities, revenues and expenses, and the disclosure of contingent assets and liabilities in the Company’s consolidated financial statements and accompanying notes. The significant estimates in the Company’s consolidated financial statements relate to the valuation of equity awards and warrant liabilities, recoverability of deferred tax assets, estimated tax credit receivable and estimated useful lives of fixed assets. The Company bases estimates and assumptions on historical experience, when available, and on various factors that it believes to be reasonable under the circumstances. The Company evaluates its estimates and assumptions on an ongoing basis, and its actual results may differ from estimates made under different assumptions or conditions.
Concentrations of Credit Risk
Financial
instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash deposited in
accounts held at two U.S. financial institutions, which may, at times, exceed federally insured limits of $
Foreign Currency Transactions
The Company and its subsidiaries use the U.S. dollar as functional currency. Foreign currency transactions are initially measured and recorded in the functional currency using the exchange rate on the date of the transaction. Foreign exchange gains and losses arising from settlement of foreign currency transactions are recognized in profit and loss.
Cocrystal Australia maintains its records in Australian dollars. The monetary assets and liabilities of Cocrystal Australia are remeasured into the functional currency using the closing rate at the end of every reporting period. All nonmonetary assets and liabilities and related profit and loss accounts are remeasured into the functional currency using the historical exchange rates. Profit and loss accounts, other than those that are remeasured using the historical exchange rates, are remeasured into the functional currency using the average exchange rate for the period. Foreign exchange gains and losses arising from the remeasurement into the functional currency is recognized in profit and loss.
Fair Value Measurements
FASB Accounting Standards Codification (“ASC”) 820 defines fair value, establishes a framework for measuring fair value under U.S. GAAP and enhances disclosures about fair value measurements. Fair value is defined under ASC 820 as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value under ASC 820 must maximize the use of observable inputs and minimize the use of unobservable inputs. The standard describes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value which are the following:
| Level 1 — quoted prices in active markets for identical assets or liabilities. | |
| Level 2 — other significant observable inputs for the assets or liabilities through corroboration with market data at the measurement date. | |
| Level 3 — significant unobservable inputs that reflect management’s best estimate of what market participants would use to price the assets or liabilities at the measurement date. |
| F-6 |
At June 30, 2024 and December 31, 2023, the carrying amounts of financial assets and liabilities, such as cash, other current assets, and accounts payable and accrued expenses approximate their fair values due to their short-term nature. The carrying values of leases payable approximate their fair values due to the fact that the interest rates on these obligations are based on prevailing market interest rates.
Long-Lived Assets
The Company regularly reviews the carrying value and estimated lives of its long-lived assets, including property and equipment, to determine whether indicators of impairment may exist which warrant adjustments to carrying values or estimated useful lives. The determinants used for this evaluation include management’s estimate of the asset’s ability to generate positive income from operations and positive cash flow in future periods as well as the strategic significance of the assets to the Company’s business objective. Should an impairment exist, the impairment loss would be measured based on the excess of the carrying amount over the asset’s fair value.
Research and Development Expenses
Research and development costs consist primarily of fees paid to consultants and outside service providers, and other expenses relating to the acquisition, design, development and testing of the Company’s clinical products. All research and development costs are expensed as incurred. Research and development costs are presented net of tax credits.
The
Company’s Australian subsidiary is entitled to receive government assistance in the form of refundable and non-refundable research
and development tax credits (“Refundable Tax Credits”) from the federal and provincial taxation authorities, based on qualifying
expenditures incurred during the fiscal year. The Refundable Tax Credits are from the provincial taxation authorities and are not dependent
on its ongoing tax status or tax position and accordingly are not considered part of income taxes. The Company records Refundable Tax
Credits as a reduction of research and development expenses when the Company can reasonably estimate the amounts and it is more likely
than not, they will be received. As of December 31, 2023, balance of Refundable Tax Credits was approximately $
Income Taxes
The Company accounts for income taxes under the asset and liability method. Under this method, deferred tax assets and liabilities are determined based on differences between financial reporting and tax bases of assets and liabilities and are measured using enacted tax rates and laws that are expected to be in effect when the differences are expected to be recovered or settled. Realization of deferred tax assets is dependent upon future taxable income. A valuation allowance is recognized if it is more likely than not that some portion or all of a deferred tax asset will not be realized based on the weight of available evidence, including expected future earnings. The Company recognizes an uncertain tax position in its financial statements when it concludes that a tax position is more likely than not to be sustained upon examination based solely on its technical merits. Only after a tax position passes the first step of recognition will measurement be required. Under the measurement step, the tax benefit is measured as the largest amount of benefit that is more likely than not to be realized upon effective settlement. This is determined on a cumulative probability basis. The full impact of any change in recognition or measurement is reflected in the period in which such change occurs. The Company elects to accrue any interest or penalties related to income taxes as part of its income tax expense.
| F-7 |
As
of June 30, 2024, the Company assessed its income tax expense based on its projected future taxable income for the year ending December
31, 2024 and therefore recorded
The Company recognizes compensation expense using a fair value-based method for costs related to stock-based payments, including stock options. The fair value of options awarded to employees is measured on the date of grant using the Black-Scholes option pricing model and is recognized as expense over the requisite service period on a straight-line basis.
Use of the Black-Scholes option pricing model requires the input of subjective assumptions including expected volatility, expected term, and a risk-free interest rate. The Company estimates volatility using a blend of its own historical stock price volatility as well as that of market comparable entities since the Company’s common stock has limited trading history and limited observable volatility of its own. The expected term of the options is estimated by using the SEC Staff Bulletin No. 107’s Simplified Method for Estimate Expected Term. The risk-free interest rate is estimated using comparable published federal funds rates.
Common Stock Purchase Warrants and Other Derivative Financial Instruments
We classify as equity any contracts that require physical settlement or net-share settlement or provide us a choice of net-cash settlement or settlement in our own shares (physical settlement or net-share settlement) provided that such contracts are indexed to our own stock as defined in ASC 815-40, Contracts in Entity’s Own Equity. We classify as assets or liabilities any contracts that require net-cash settlement (including a requirement to net cash settle the contract if an event occurs and if that event is outside our control) or give the counterparty a choice of net-cash settlement or settlement in shares (physical settlement or net-share settlement). We assess classification of our common stock purchase warrants and other freestanding derivatives at each reporting date to determine whether a change in classification between assets and liabilities is required.
The Company accounts for and discloses net income (loss) per common share in accordance with FASB ASC Topic 260, Earnings Per Share. Basic income (loss) per common share is computed by dividing income (loss) attributable to common stockholders by the weighted average number of common shares outstanding. Diluted net income (loss) per common share is computed by dividing net income (loss) attributable to common stockholders by the weighted average number of common shares that would have been outstanding during the period assuming the issuance of common stock for all potential dilutive common shares outstanding. Potential common shares consist of shares issuable upon the exercise of stock options and warrants and the conversion of convertible notes payable.
| June 30, | ||||||||
| 2024 | 2023 | |||||||
| Outstanding options to purchase common stock | ||||||||
| Warrants to purchase common stock | ||||||||
| Total | ||||||||
| F-8 |
Recent Accounting Pronouncements
Authoritative guidance issued by the FASB (including technical corrections to the ASC), the American Institute of Certified Public Accountants, and the SEC did not, or are not expected to, have a material impact on the Company’s consolidated financial statements and related disclosures.
3. Property and Equipment
Property
and equipment are recorded at cost and depreciated over the estimated useful lives of the underlying assets ( to
| June 30, 2024 | December 31, 2023 | |||||||
| Lab equipment (excluding equipment under finance leases) | $ | $ | ||||||
| Finance lease right-of-use lab equipment obtained in exchange for finance lease liabilities, net | ||||||||
| Computer and office equipment | ||||||||
| Total property and equipment | ||||||||
| Less: accumulated depreciation and amortization | ( | ) | ( | ) | ||||
| Property and equipment, net | $ | $ | ||||||
Total
depreciation and amortization expense were approximately $
4. Accounts Payable and Accrued Expenses
Accounts payable and accrued expenses consisted of the following (in thousands) as of:
| June 30, 2024 | December 31, 2023 | |||||||
| Accounts payable | $ | $ | ||||||
| Accrued compensation | ||||||||
| Accrued other expenses | ||||||||
| Total accounts payable and accrued expenses | $ | $ | ||||||
Accounts payable and accrued other expenses contain unpaid general and administrative expenses and costs related to research and development that have been billed and estimated unbilled, respectively, as of period-end.
5. Common Stock and Preferred Stock
As of June 30, 2024, the Company has authorized shares of common stock, $ par value per share, and shares of preferred stock, $ par value per share.
On
June 27, 2024, the Company, following approval of the Company’s stockholders at the 2024 Annual Meeting of Stockholders filed an
amendment to its Certificate of Incorporation with the Secretary of State of the State of Delaware (the “Amendment”) to decrease
the number of shares of authorized capital stock of the Company from
The Company had shares of common stock and shares of preferred stock issued and outstanding as of June 30, 2024, and December 31, 2023.
| F-9 |
Equity Incentive Plans
The Company adopted an equity incentive plan in 2015 (the “2015 Plan”) under which shares of common stock have been reserved for issuance to employees, and non-employee directors and consultants of the Company. Recipients of incentive stock options granted under the 2015 Plan shall be eligible to purchase shares of the Company’s common stock at an exercise price equal to no less than the estimated fair market value of such stock on the date of grant. The maximum term of options granted under the 2015 Plan is . On June 16, 2021, the Company’s stockholders voted to approve an amendment to the 2015 Plan to increase the number of shares of common stock authorized for issuance under the 2015 Plan from to shares. As of June 30, 2024, shares remain available for future grants under the 2015 Plan.
| Number of Shares Available for Grant | Total Options Outstanding | Weighted Average Exercise Price | Aggregate Intrinsic Value | |||||||||||||
| Balance at December 31, 2023 | $ | $ | ||||||||||||||
| Exercised | ||||||||||||||||
| Granted | ||||||||||||||||
| Expired | ( | ) | ||||||||||||||
| Balance at June 30, 2024 | $ | $ | ||||||||||||||
The Company accounts for share-based awards to employees and nonemployee directors and consultants in accordance with the provisions of ASC 718, Compensation—Stock Compensation., and under the recently issued guidance following FASB’s pronouncement, ASU 2018-07, Compensation—Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting. Under ASC 718, and applicable updates adopted, share-based awards are valued at fair value on the date of grant and that fair value is recognized over the requisite service, or vesting, period. The Company values its equity awards using the Black-Scholes option pricing model, and accounts for forfeitures when they occur. For the three and six months ended June 30, 2024 and 2023, equity-based compensation expense recorded was approximately and $ and $ and $, respectively.
As of June 30, 2024, there was approximately $ of total unrecognized compensation expense related to non-vested stock options that is expected to be recognized over a weighted average period of years. For options granted and outstanding, there were options outstanding which were fully vested or expected to vest, with an aggregate intrinsic value of $, a weighted average exercise price of $ and weighted average remaining contractual term of years at June 30, 2024. For vested and exercisable options, outstanding shares totaled , with an aggregate intrinsic value of $. These options had a weighted average exercise price of $per share and a weighted-average remaining contractual term of years at June 30, 2024.
The aggregate intrinsic value of outstanding and exercisable options at June 30, 2024 was calculated based on the closing price of the Company’s common stock as reported on The Nasdaq Capital Market on June 30, 2024 of $ per share less the exercise price of the options. The aggregate intrinsic value is calculated based on the positive difference between the closing fair market value of the Company’s common stock and the exercise price of the underlying options.
| F-10 |
Common Stock Reserved for Future Issuance
The following table presents information concerning common stock available for future issuance (in thousands) as of:
| June 30, 2024 | June 30, 2023 | |||||||
| Stock options issued and outstanding | ||||||||
| Shares authorized for future option grants | ||||||||
| Warrants outstanding | ||||||||
| Total | ||||||||
7. Warrants
The following is a summary of activity in the number of warrants classified as liabilities outstanding to purchase the Company’s common stock for the six months ended June 30, 2024 (in thousands):
| January 2014 Warrants | ||||
| Outstanding, December 31, 2023 | ||||
| Exercised | ||||
| Granted | ||||
| Expired | ( | ) | ||
| Outstanding, June 30, 2024 | ||||
| Expiration date: | ||||
8. Licenses and Collaborations
Merck Sharp & Dohme Corp.
On
January 2, 2019, the Company entered into an Exclusive License and Research Collaboration Agreement (the “Collaboration Agreement”)
with Merck Sharp & Dohme LLC (“Merck”) to discover and develop certain proprietary influenza A/B antiviral agents. Under
the terms of the Collaboration Agreement, Merck funded research and development for the program, including clinical development, and
was responsible for worldwide commercialization of any products derived from the collaboration. Under the Collaboration Agreement Cocrystal
was eligible to receive payments related to designated development, regulatory and sales milestones with the potential to earn up to
$
On December 15, 2023, the Company received written notice from Merck of Merck’s election to terminate the Collaboration Agreement. The termination of the Collaboration Agreement took effect on March 14, 2024. According to Merck’s termination notice, Merck determined there were no existing conditions to continue the collaboration. The termination resulted from the inability to develop the compounds to meet a specific aspect of Merck’s program. The pending patent applications on compounds covered by the Collaboration Agreement and previously filed by Merck on behalf of both companies remain in place.
Kansas State University Research Foundation
Cocrystal entered into two License Agreement with Kansas State University Research Foundation (the “Foundation”) on February 18, 2020 to further develop certain proprietary broad-spectrum antiviral compounds for the treatment of norovirus and coronavirus infections.
| F-11 |
On February 28, 2024, the Company provided notice to the Foundation of the Company’s election to terminate the 2020 License Agreements. The terminations, which were made due to the Company’s determination that further development efforts under the License Agreements would be futile, took effect on March 29, 2024.
9. Commitments and Contingencies
Commitments
In the ordinary course of business, the Company enters into non-cancellable leases to purchase equipment and for its facilities, including related party leases (see Note 10 – Transactions with Related Parties). Leases are accounted for as operating leases or finance leases, in accordance with ASC 842, Leases.
Operating Leases
The
Company leases office space in Miami, Florida and research and development laboratory space in Bothell, Washington under operating leases
that expire on
The following table summarizes the Company’s maturities of operating lease liabilities, by year and in aggregate, as of June 30, 2024 (table in thousands):
| 2024 (excluding the six months ended June 30, 2024) | $ | |||
| 2025 | ||||
| 2026 | ||||
| 2027 | ||||
| 2028 | ||||
| 2029 and thereafter | ||||
| Total operating lease payments | ||||
| Less: present value discount | ( | ) | ||
| Total operating lease liabilities | $ |
As
of June 30, 2024, the total operating lease liability of $
The
operating lease liabilities summarized above do not include variable common area maintenance (the “CAM”) charges, which are
contractual liabilities under the Company’s Bothell, Washington lease. CAM charges for the Bothell, Washington facility are calculated
annually based on actual common expenses for the building incurred by the lessor and proportionately billed to tenants based on leased
square footage. For the six months ended June 30, 2024 and 2023, approximately $
The minimum lease payments above include the amounts that would be paid if the Company maintains its Bothell lease for the five-year term, starting February 2024.
On
September 1, 2021, the Company entered into a three-year lease extension with a limited liability company controlled by Dr. Phillip Frost,
a director and a principal stockholder of the Company. On an annualized basis, straight-line rent expense is approximately $
On
September 21, 2023, the Company amended the lease agreement with a North Creek Tec LLC, to expand its laboratory facility in Bothell
– WA, with additional
| F-12 |
For
the six months ended June 30, 2024 and 2023, operating lease expense, excluding short-term leases, finance leases and CAM charges, totaled
approximately $
Finance Leases
In
April 2020, the Company entered into lease agreements to acquire lab equipment with
The
leased lab equipment is depreciable over
Phase 2a Clinical Trial
On
August 3, 2022 the Company engaged hVIVO, a subsidiary of London-based Open Orphan plc (AIM: ORPH), a rapidly growing specialist contract
research organization (“CRO”), to conduct a Phase 2a clinical trial with the Company’s novel, broad-spectrum, orally
administered antiviral influenza candidate. The Company prepaid a reservation fee of $
The
total estimated cost of the agreement (including the reservation fee) is approximately $
On
May 21, 2024, the Company entered into a new agreement with hVIVO, as a follow-on to CPI-CST-001 Influenza virus challenge study, in
which potential resistance to CC-42344 antiviral compound will be genotypically characterized. The Company incurred $
Contingencies
From time to time, the Company is a party to, or otherwise involved in, legal proceedings arising in the normal course of business. As of the date of this report, except as described below, the Company is not aware of any proceedings, threatened or pending, against it which, if determined adversely, would have a material effect on its business, results of operations, cash flows or financial position.
10. Transactions with Related Parties
On
September 1, 2021, the Company entered into a
On
April 4, 2023, the Company entered into a Securities Purchase Agreement with two accredited investors (the “Purchasers”)
whereby the Purchasers agreed to purchase a total of shares of unregistered common stock at a price of $ per share for
a total purchase price of $
| F-13 |
ITEM 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Overview
Cocrystal Pharma, Inc. (the “Company” or “Cocrystal”) is a clinical-stage biotechnology company seeking to discover and develop novel antiviral therapeutics as treatments for serious and/or chronic viral diseases. We employ unique structure-based technologies and Nobel Prize winning expertise to create first- and best-in-class antiviral drugs. These technologies are designed to efficiently deliver small molecule therapeutics that are safe, effective and convenient to administer. We have identified promising preclinical and clinical-stage antiviral compounds for unmet medical needs including influenza virus, coronavirus, norovirus and hepatitis C virus (“HCV”).
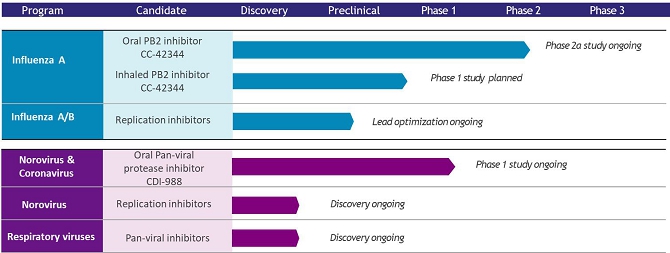
Research and Development Update
During the six months ended June 30, 2024 and more recently the Company continued to focus its research and development efforts primarily in three areas. The following is an overview of the characteristics, goals and development progress of each of these programs.

Influenza Program
We have several candidates under development for the treatment of influenza infection. CC-42344, a novel PB2 inhibitor, was selected as a preclinical lead for the treatment of pandemic and seasonal influenza A. Oral CC-42344 was advanced to a Phase 2a influenza human challenge clinical study as described in more detail below. This drug candidate binds to a highly conserved PB2 site of influenza polymerase complex (PB1: PB2: PA) and exhibits a novel mechanism of action. CC-42344 showed excellent antiviral activity against influenza A strains, including avian pandemic strains and strains resistant to Tamiflu® and Xofluza, and has favorable pharmacokinetic and drug resistance profiles. This drug candidate was specifically designed and developed using Cocrystal’s proprietary structure-based drug discovery platform technology.
We received authorization from the United Kingdom Medicines and Healthcare Products Regulatory Agency (MHRA) to initiate a Phase 2a human challenge study with oral CC-42344 as a potential treatment for pandemic and seasonal influenza A. This ongoing randomized, double-blind, placebo-controlled study is evaluating the safety, tolerability, viral and clinical measurements of influenza A infection in subjects dosed with oral CC-42344 treatment. In May 2024 we announced completion of enrollment of 78 subjects.
| 3 |
In June 2024 we reported the potential efficacy of CC-42344 against the new avian flu strain with the recently published genome sequence for H5N1. Using our proprietary structure-based platform technology, Cocrystal created a high-resolution crystal structure of this avian PB2 protein and confirmed that CC-42344 binds to its highly conserved PB2 region. The in vitro data generated testing CC-42344 against the avian H5N1 PB2 protein showed a desired level of CC-42344’s activity similar to that of Cocrystal’s data in other strains of pandemic and seasonal influenza A.
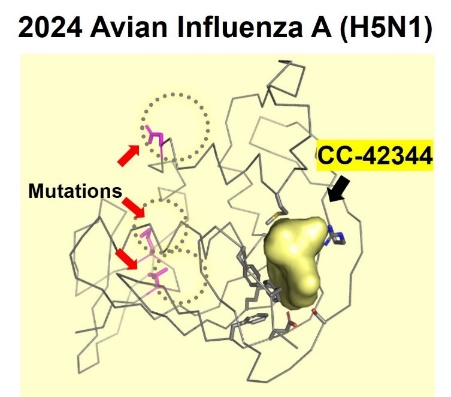
CC-42344 binds to the highly conserved region of the avian influenza A H5N1 PB2 protein
In addition to oral CC-42344, we developed inhaled CC-42344 for the prophylactic treatment of pandemic and seasonal influenza A infections. Our preclinical data of inhaled CC-42344 showed excellent antiviral activity in influenza H1N1-infected human upper airway epithelium with a favorable safety profile. We completed inhalation formulation development and are conducting 14-day GLP toxicology study.
We also continue developing novel broad-spectrum influenza antivirals targeting replication enzymes of influenza A and B strains.
Norovirus and Coronavirus Programs
We developed novel protease inhibitor CDI-988 as an oral pan-viral treatment of noroviruses and coronavirus, including SARS-CoV-2 and its variants. CDI-988 was specifically designed and developed using our proprietary structure-based drug discovery platform technology as a broad-spectrum antiviral inhibitor to a highly conserved region in the active site of noroviruses, coronaviruses and other 3CL viral proteases.
Oral CDI-988 is being clinically evaluated for safety, tolerability and pharmacokinetics including a food-effect cohort in healthy volunteers in a single-center, randomized, double-blind, placebo-controlled Phase 1 study being conducted in Australia.
In July 2024 we announced favorable safety and tolerability results from the single-ascending dose (SAD) cohorts of the Phase 1 study with CDI-988. Study participants in the SAD cohorts received CDI-988 in doses ranging from 100 mg to 600 mg. All participants completed the study with no discontinuations. There were no serious adverse events or severe treatment-emergent adverse events. No clinically significant observations were noted in laboratory assessments, physical exams or electrocardiograms.
Therapeutic Targets
Influenza: A worldwide public health problem, including the potential for pandemic disease.
Influenza is a severe respiratory illness, caused primarily by influenza A or B virus. Influenza A viruses are the only influenza viruses known to cause influenza pandemics. Each year there are approximately 1 billion cases of seasonal influenza worldwide, with 3-5 million severe illnesses and up to 650,000 deaths, according to the World Health Organization (“WHO”). On average about 8% of the U.S. population contracts influenza each season, according to the Centers for Disease Control and Prevention (“CDC”). In addition to the health risk, influenza is responsible for approximately $10.4 billion in direct medical costs in the U.S. annually, according to the National Institutes of Health (“NIH”).
| 4 |
Currently, approved antiviral treatments for influenza are effective, but burdened with significant viral resistance. Strains of influenza virus that are resistant to the approved treatments oseltamivir phosphate (Tamiflu®), zanamavir (Relenza®) and baloxavir marboxil (Xofluza®) have appeared, and in some cases are predominant. For example, the predominant strain of the 2009 swine influenza pandemic was resistant to oseltamivir. Oseltamivir inhibits influenza neuraminidase enzymes, which are not highly conserved between viral strains. According to the WHO, approximately 15% of the H1N1 isolated circulating worldwide were oseltamivir resistant. Also, treatment-emergent resistance to recently approved baloxavir has been observed during clinical trials and the potential transmission of resistant influenza variants could significantly diminish baloxavir effectiveness.
Coronavirus: COVID-19 continues to be a global pandemic fueled by an emergence of new strains.
COVID-19 is a global pandemic with 775,673,955 confirmed cases globally, including 7,053,524 deaths, as of July 7, 2024, according to data reported by the WHO.
Coronaviruses (CoV) are a large family of RNA viruses that historically have been associated with illness ranging from mild symptoms similar to the common cold to more severe respiratory disease. Infection with the novel SARS-CoV-2 has been associated with a wide range of responses, from no symptoms to more severe disease that has included pneumonia, severe acute respiratory syndrome, kidney failure, and death. The incubation period for SARS-CoV-2 is believed to be within 14 days after exposure, with most illness occurring within about five days after exposure. SARS-CoV-2, like other RNA viruses, is prone to mutate over time, resulting in the emergence of multiple variants. Adaptive mutations in the viral genome can alter the virus’s pathogenic potential. Even a single amino acid exchange can drastically affect a virus’s ability to evade the immune system and complicate the vaccine and antibody therapeutics development against the virus. Based on the recent epidemiological update by the WHO, five SARS-CoV-2 VOCs (variants of concern) have been identified since the beginning of the pandemic. Also, as demonstrated in Delta and Omicron variants as well as the more recent JN.1 strain, some variations allow the virus to spread more easily and make it resistant to the treatments and vaccines.
On October 22, 2020, the U.S. Food and Drug Administration (“FDA”) approved the antiviral drug Veklury® (remdesivir) for the treatment of COVID-19 requiring hospitalization. Remdesivir is a nucleotide prodrug that inhibits viral replication and was previously evaluated in clinical trials for Ebola treatment in 2014. On May 25, 2023, the FDA approved Paxlovid™ (nirmatrelvir tablets and ritonavir tablets, co-packaged for oral use) for use to treat COVID-19 for the treatment of mild-to-moderate COVID-19 in adults who are at high risk for progression to severe COVID-19, including hospitalization or death. For certain hospitalized adults with COVID-19, the FDA has also approved Olumiant® (baricitinib) and Actemra® (tocilizumab). In addition, the FDA issued emergency use authorization (EUA) for several antibody and antiviral therapeutics, including and Lagevrio™ (molnupiravir).
We continue pursuing the development of novel antiviral compounds for the treatment of coronavirus infections using our established proprietary drug discovery platform. By targeting the viral replication enzymes and protease, we believe it is possible to develop an effective treatment for all coronavirus diseases including COVID-19, Severe Acute Respiratory Syndrome (SARS), and Middle East Respiratory Syndrome (MERS).
Norovirus: A worldwide public health problem responsible for close to 90% of epidemic, non-bacterial outbreaks of gastroenteritis around the world with no effective treatment.
Norovirus is a very common and highly contagious virus that causes symptoms of acute gastroenteritis among people of all ages. Norovirus infection can be significantly more severe and prolonged in specific risk groups including infants, children, the elderly and people with immunodeficiency. Symptoms include nausea, vomiting, stomach pain and diarrhea as well as fatigue, fever and dehydration. Outbreaks occur most commonly in semi-closed communities and have become notorious for their occurrence in hospitals, nursing homes, childcare facilities, cruise ships, schools, disaster relief sites and military settings. In the U.S. alone, noroviruses are responsible for an estimated 21 million cases annually, including 109,000 hospitalizations, 465,000 emergency department visits and nearly 900 deaths, according to the CDC. The NIH estimates the annual burden to the United States at $10.6 billion. Noroviruses are responsible for up to 1.1 million hospitalizations and 218,000 deaths annually in children in the developing world. In immunosuppressed patients, chronic norovirus infection can lead to a debilitating illness with extended periods of nausea, vomiting and diarrhea.
| 5 |
There is currently no effective treatment or effective vaccine for norovirus, and the ability to curtail outbreaks is limited. We have a norovirus therapeutic candidate in clinical testing. A few companies have been developing vaccines and are in stages of clinical testing, including Vaxart Pharmaceutical, Moderna, Hillevax, Takeda Pharmaceuticals, Anhui Zhifei Longcom Biopharmaceutical (China) and National Vaccine and Serum Institute (China).
By targeting viral replication enzymes and a viral protease, we believe it is possible to develop an effective treatment for all genogroups of norovirus. Also, because of the significant unmet medical need and the possibility of chronic norovirus infection in immunocompromised individuals, new antiviral therapeutic and prophylactic approaches may warrant an accelerated path to market. We are developing inhibitors of the RNA-dependent RNA polymerase and protease of norovirus. These enzymes are essential to viral replication and are highly conserved between all noroviral genogroups. Therefore, an inhibitor of these enzymes might be an effective treatment or short-term prophylactic agent, when administered during a cruise or nursing home stay, for example. We have developed X-ray quality norovirus polymerase and protease crystals and have identified promising inhibitors. We are implementing the platform and approaches that have proven successful in our other antiviral programs.
Hepatitis C: A large competitive market with opportunity for shorter treatment regimens.
HCV is a highly competitive and changing market. Since 2014, several combinations of direct-acting antiviral agents (“DAAs”) have been approved for the treatment of HCV infection. These include Harvoni® (sofosbuvir/ledipasvir) 12 weeks of treatment, Viekira Pak™ (ombitasvir/paritaprevir/ritonavir, dasabuvir) 12 weeks of treatment, Epclusa® (sofosbuvir/velpatasvir) 12 weeks of treatment, Zepatier™ (elbasvir/grazoprevir) 12 weeks of treatment and Mavyret® (glecaprevir/pibrentasvir) eight weeks of treatment. We believe the next improvements in HCV treatment will be ultra-short combination oral treatments of four to six weeks, which is the goal of our program.
We anticipate a significant global HCV market opportunity that will persist through at least 2036, given the large prevalence of HCV infection worldwide. The 2024 World Health Organization Global Hepatitis Report estimates that 50 million people worldwide have chronic HCV infections with about 1 million new infections occurring per year and an estimated 3.2 million adolescents and children with chronic HCV infection.
We are targeting the viral NS5B polymerase with an NNI, which could be developed as part of an all-oral, pan-genotypic combination regimen. Our focus is on developing what is now called ultrashort treatment regimens from four to six weeks in length. Combining CC-31244 with different classes of approved DAAs has the potential to change the paradigm of treatment for HCV by shortening the duration of treatment. Combination strategies with approved drugs could allow us to expand CC-31244 into the HCV antiviral therapeutic area globally and could lead to a high and fast cure rate, to improved compliance, and to reduced treatment duration. To our knowledge no competing company has yet developed a short HCV treatment of less than 8 weeks with a high (>95%) sustained virologic response (SVR) at week 12.
CC-31244, an HCV NNI, is a potential best in class pan-genotypic inhibitor of NS5B polymerase for the treatment of HCV. We completed a randomized, double-blinded Phase 1a/b study in healthy volunteers and HCV-infected subjects in Canada in September 2016, with favorable safety results. We completed a Phase 2a study in HCV genotype 1 subjects in the U.S. in 2017. HCV-infected subjects treated with CC-31244 had a rapid and marked decline in HCV RNA levels, and slow viral rebound after treatment. Results of this study suggest that CC-31244 could be an important component in a shortened duration all-oral HCV combination therapy. We have completed the Phase 2a final study report as filed with the FDA. See “Item 1 – Business – Research and Development Update – Hepatitis C” in our Annual Report on Form 10-K for the year ended December 31, 2023 for more information.
We have been seeking a partner for further clinical development of CC-31244 since completing Phase 2a trials.
| 6 |
Results of Operations for the Six Months Ended June 30, 2024 compared to the Six Months Ended June 30, 2023
Research and Development Expense
Research and development expense consists primarily of compensation-related costs for our employees dedicated to research and development activities and clinical trials, as well as lab supplies, lab services, and facilities and equipment costs related to our research and development programs.
Total research and development expenses for the three months ended June 30, 2024, and 2023 were $4,308,000 and $2,801,000, respectively. The increase of $1,507,000 was primarily due to our Influenza CC-42344 product candidate entering into a Phase 2a clinical trial and our norovirus and coronavirus candidate CDI-988 entering into a Phase 1 clinical trial.
Total research and development expenses for the six months ended June 30, 2024, and 2023 were $7,258,000 and $6,708,000, respectively. The increase of $550,000 was primarily due ongoing clinical trials described above.
General and Administrative Expense
General and administrative expenses include compensation-related costs for our employees dedicated to general and administrative activities, legal fees, audit and tax fees, consultants and professional services, and general corporate expenses.
General and administrative expenses for the three months ended June 30, 2024, and 2023 were $1,140,000 and $1,538,000, respectively. The decrease of $398,000 was primarily due to a reduction of litigation expenses.
General and administrative expenses for the six months ended June 30, 2024, and 2023 were $2,348,000 and $2,742,000, respectively. The decrease of $394,000 was primarily due to a reduction of litigation expenses.
Interest Income, Net
Interest income for the three months ended June 30, 2024 and 2023 was $151,000 and $140,000, respectively, and for the six months ended June 30, 2024 and 2023 was $371,000 and $140,000, respectively. The interest income was primarily earned on cash held in interest bearing bank accounts.
Foreign Exchange Loss
In 2022, the Company established a wholly owned subsidiary in Australia, making it subject to foreign exchange rate fluctuations. Foreign exchange loss during the six months ended June 30, 2024, and 2023 was $64,000 and $45,000, respectively.
Income Taxes
No income tax benefit or expense was recognized for the three and six months ended June 30, 2024 and 2023. The Company’s effective income tax rate was 0.00% and 0.00% for the three and six months ended June 30, 2024 and 2023. As a result of the Company’s cumulative losses, management has concluded that a full valuation allowance against the Company’s net deferred tax assets is appropriate.
Net Loss
As a result of the above factors, net loss for the three and six months ended June 30, 2024 was $5,343,000 and $9,299,000, compared with a net loss of $4,166,000 and $9,355,000 for the three and six months ended June 30, 2023, respectively, primarily as a result of operations described above.
| 7 |
Liquidity and Capital Resources
Net cash used in operating activities was $8,202,000 for the six months ended June 30, 2024 compared with net cash used in operating activities of $8,659,000 for the same period in 2023. This decrease was primarily due to period expenses related to the completion of our COVID-19 Phase 1 clinical trial and completion of our Influenza A Phase 2a clinical trial and preparation for our anticipated Influenza A Phase 1 inhaler administer medicine clinical trial and completion of our COVID-19 Phase 1 clinical trial.
We used $8,000 net cash for investing activities during the six months ended June 30, 2024 compared with $59,000 net cash used for the same period in 2023. For the six months ended June 30, 2024 the level of investments decreased compared with June 30, 2023 due to comparative reduction in purchases of laboratory equipment in 2024.
Net cash used in financing activities totaled $0 for the six months ended June 30, 2024 compared with net cash used in financing activities of $3,993,000 for the same period in 2023 due to sufficient capital resulting in a lack of financing activity in the 2024 period.
The Company has not yet established an ongoing source of revenue sufficient to cover its operating costs. The Company had $18,143,000 unrestricted cash on June 30, 2024. The Company believes it has sufficient cash to maintain planned operations for more than the next 12 months.
We have focused our efforts on research and development activities, including through collaborations with suitable partners. We have been profitable on a quarterly basis but have never been profitable on an annual basis. We have no products approved for sale and have incurred operating losses and negative operating cash flows on an annual basis since inception.
The Company’s interim consolidated financial statements are prepared using generally accepted accounting principles in the United States of America applicable to a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. Historically, public and private equity offerings have been our principal source of liquidity.
The Company is party to the At-The-Market Offering Agreement, dated July 1, 2020 (“ATM Agreement”) with H.C. Wainwright & Co., LLC (“Wainwright”), pursuant to which the Company may issue and sell over time and from time to time, to or through Wainwright, up to $10,000,000 of shares of the Company’s common stock. During January 2021, the Company sold 1,030,000 shares of its common stock pursuant to the ATM Agreement for net proceeds of approximately $2,072,000. On May 24, 2023, the Company filed a prospectus supplement covering sales under the ATM Agreement under which we may offer and sell shares of our common stock having an aggregate offering price of up to $7,250,000 from time to time through Wainwright. There were no sales under the ATM Agreement during the six months ended June 30, 2024.
As the Company continues to incur losses, achieving profitability is dependent upon the successful development, approval and commercialization of its product candidates, and achieving a level of revenues adequate to support the Company’s cost structure. The Company may never achieve profitability, and unless and until it does, the Company will continue to need to raise additional capital. Management intends to fund future operations through additional private or public equity offerings and through arrangements with strategic partners or from other sources. There can be no assurances, however, that additional funding will be available on terms acceptable to the Company, or at all, and any equity financing may be very dilutive to existing stockholders.
| 8 |
Cautionary Note Regarding Forward-Looking Statements
This report includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, including statements regarding the future effectiveness of our product candidates, our plans for the future development of preclinical and clinical drug candidates, the progress and expected or potential timelines of achieving certain value driving milestones in our programs, progressing our programs in the clinical development process generally, our expectations regarding future operating results and liquidity. The words “believe,” “may,” “estimate,” “continue,” “anticipate,” “intend,” “should,” “plan,” “could,” “target,” “potential,” “is likely,” “will,” “expect” and similar expressions, as they relate to us, are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy and financial needs.
The results anticipated by any or all of these forward-looking statements might not occur. Important factors that could cause actual results to differ from those in the forward-looking statements include the risks and uncertainties arising from the risks arising from inflation, interest rate increases, the possibility of a recession and the economic impact of the wars in Israel and Ukraine on our Company, our collaboration partners, and on the U.S., U.K., Australia and global economies, including downturns in economic activity and capital markets, manufacturing and research delays arising from raw materials and labor shortages, supply chain disruptions and other business interruptions including any adverse impacts on our ability to obtain raw materials and test animals as well as similar problems with our vendors and our current and any future contract research organizations (CROs) and contract manufacturing organizations (CMOs), the ability of our CROs to recruit volunteers for, and to proceed with, clinical studies, and our collaboration partners’ technology and software performing as expected, financial difficulties experienced by certain partners, the results of the studies for CC-42344 and CDI-988 and any future preclinical and clinical trials we or our strategic partners undertake, general risks arising from clinical trials, receipt of regulatory approvals, regulatory changes, development of effective treatments and/or vaccines by competitors, including as part of the programs financed by governmental authorities and potential mutations in a virus we are targeting which may result in variants that are resistant to a product candidate we develop. Further information on our risk factors is contained in our filings with the SEC, including our Annual Report on Form 10-K for the year ended December 31, 2023. We undertake no obligation to publicly update or revise any forward-looking statements, whether as the result of new information, future events or otherwise.
Critical Accounting Policies and Estimates
In our Annual Report on Form 10-K for the year ended December 31, 2023, we disclosed our critical accounting policies and estimates upon which our financial statements are derived.
Accounting estimates. The preparation of financial statements in conformity with accounting principles generally accepted in the U.S. requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ significantly from these estimates.
Readers are encouraged to review these disclosures in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023 in conjunction with the review of this report.
ITEM 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not applicable.
ITEM 4. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
We carried out an evaluation, under the supervision and with the participation of our management, including our Co-Chief Executive Officers and Chief Financial Officer, of the effectiveness of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934 (the “Exchange Act”) as of the end of the period covered by this report. Based on that evaluation, our Co-Chief Executive Officers and Chief Financial Officer have concluded that our disclosure controls and procedures as of June 30, 2024 were effective to ensure that information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms.
Changes in Internal Control over Financial Reporting
There were no material changes in our internal controls over financial reporting or in other factors that could materially affect, or are reasonably likely to affect, our internal controls over financial reporting during the quarter ended June 30, 2024. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
| 9 |
PART II — OTHER INFORMATION
ITEM 1. LEGAL PROCEEDINGS
From time to time, the Company is a party to, or otherwise involved in, legal proceedings arising in the normal course of business. During the reporting period, there have been no material changes to the description of legal proceedings set forth in our Annual Report on Form 10-Q for the year ended June 30, 2024.
ITEM 1.A RISK FACTORS
None.
ITEM 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
All recent sales of unregistered securities have been previously reported.
ITEM 3. DEFAULTS UPON SENIOR SECURITIES
None.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
ITEM 5. OTHER INFORMATION
During
the six months ended June 30, 2024, none of our directors or officers (as defined in Rule 16a-1(f) under the Exchange Act)
| 10 |
ITEM 6. EXHIBITS
The exhibits listed in the accompanying “Exhibit Index” are filed or incorporated by reference as part of this Form 10-Q.
EXHIBIT INDEX
| Exhibit | Incorporated by Reference | Filed or Furnished | ||||||||
| No. | Exhibit Description | Form | Date | Number | Herewith | |||||
| 3.1 | Certificate of Incorporation, as amended | 10-Q | 8/16/21 | 3.1 | ||||||
| 3.1(a) | Certificate of Amendment to Certificate of Incorporation | 8-K | 10/3/22 | 3.1 | ||||||
| 3.1(b) | Certificate of Amendment to Certificate of Incorporation – reduce number of authorized shares | 8-K | 6/28/24 | 3.1 | ||||||
| 3.2 | Amended and Restated Bylaws | 8-K | 2/19/21 | 3.1 | ||||||
| 31.1 | Certification of Principal Executive Officer (302) | Filed | ||||||||
| 31.2 | Certification of Principal Executive Officer (302) | Filed | ||||||||
| 31.3 | Certification of Principal Financial Officer (302) | Filed | ||||||||
| 32.1 | Certification of Principal Executive and Principal Financial Officer (906) | Furnished* | ||||||||
| 101.INS | Inline XBRL Instance Document | Filed | ||||||||
| 101.SCH | Inline XBRL Taxonomy Extension Schema Document | Filed | ||||||||
| 101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase Document | Filed | ||||||||
| 101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document | Filed | ||||||||
| 101.LAB | Inline XBRL Taxonomy Extension Label Linkbase Document | Filed | ||||||||
| 101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase Document | Filed | ||||||||
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | Filed | ||||||||
* This exhibit is being furnished rather than filed and shall not be deemed incorporated by reference into any filing, in accordance with Item 601 of Regulation S-K.
Copies of this report (including the financial statements) and any of the exhibits referred to above will be furnished at no cost to our stockholders who make a written request to our Corporate Secretary at Cocrystal Pharma, Inc., 4400 Biscayne Blvd, Suite 101, Miami, FL 33137.
| 11 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Cocrystal Pharma, Inc. | ||
| Dated: August 14, 2024 | By: | /s/ Sam Lee |
| Sam Lee | ||
| President and Co-Chief Executive Officer | ||
| (Principal Executive Officer) | ||
| Dated: August 14, 2024 | By: | /s/ James Martin |
| James Martin | ||
Chief Financial Officer and Co-Chief Executive Officer | ||
| (Principal Executive Officer and Principal Financial Officer) | ||
| 12 |